Biotech
Biotech and drug development research are crucial for advancing human health and wellbeing. In recent years, there have been significant advancements in our understanding of biological systems and novel biotech discovery tech, delivery mechanisms, progress in ai drug discovery and more.
One of the key challenges in drug development is identifying compounds that can effectively target a specific biological pathway or disease without causing harmful side effects. Traditionally, this process involved testing compounds on cells or animals in a laboratory setting, which is time-consuming and costly.
Recent advances in technology have enabled the use of computational modeling and simulation to predict the potential efficacy and safety of compounds. This approach allows researchers to quickly and efficiently screen large numbers of compounds and identify potential candidates for further testing. In addition to computational modeling, the use of genetically-engineered animal models has also become increasingly common in drug development research. These models allow researchers to study the effects of compounds on specific biological pathways in a more controlled and precise manner.
Despite these advancements, drug development remains a complex and challenging process. Success rates for new drug candidates are low, and the cost of bringing a new drug to market can be prohibitively expensive. However, continued investment in research and development in the biotech and drug development fields has the potential to yield significant benefits for human health.
Overall, the field of biotech and drug development research is making important contributions to our understanding of biological systems and the development of effective treatments for a wide range of diseases. By continuing to invest in research and innovation, we can improve our ability to discover and develop new drugs and enhance human health and wellbeing.
Need for metascience in biotech
Metascience, or the study of scientific research itself, is crucial for advancing our understanding the most effective approaches for drug discovery: examining the strengths and limitations of different research methods, disease models and studying the factors that contribute to successful drug development.
One of the key challenges in the field of biotech and drug research is the high cost and low success rates of drug development. Trying to better understand the factors that contribute to the success or failure of drug candidates, studying the limitations of current disease models and identify more accurate and predictive models that can be used to test the potential efficacy and safety of drug candidates. This can improve the reliability and validity of research findings and ultimately lead to the development of more effective treatments.
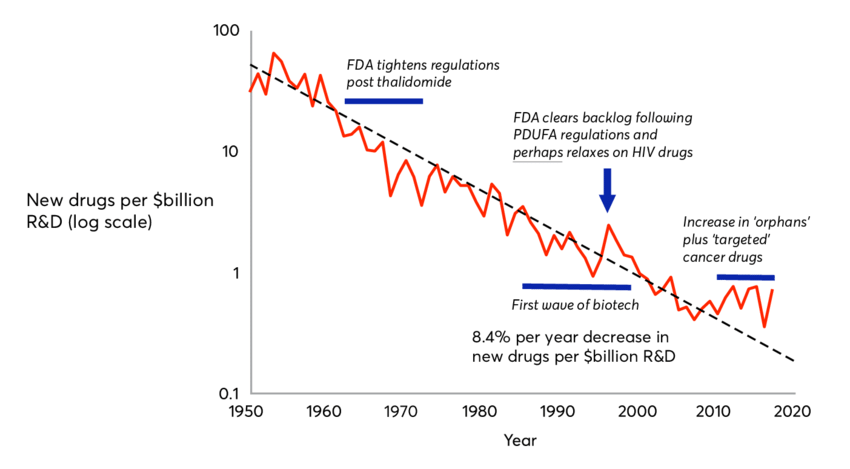
Eroom’s Law: Declining R&D Productivity
Eroom’s Law is the observation that the cost of developing a new drug has been increasing at an exponential rate since the 1950s. The average cost of developing a new drug was $320 million in 2010, up from $80 million in 1980. The new version of the law, which includes the cost of clinical trials, puts the average cost at $2.6 billion. The law has been used to explain the high cost of prescription drugs and the lack of innovation in the pharmaceutical industry.
Potential Solutions: Predictive validity in drug discovery
“Successful drug discovery is like finding oases of safety and efficacy in chemical and biological deserts. Screens in disease models, and other decision tools used in drug research and development (R&D), point towards oases when they score therapeutic candidates in a way that correlates with clinical utility in humans. Otherwise, they probably lead in the wrong direction. This line of thought can be quantified by using decision theory, in which ‘predictive validity’ is the correlation coefficient between the output of a decision tool and clinical utility across therapeutic candidates. Analyses based on this approach reveal that the detectability of good candidates is extremely sensitive to predictive validity, because the deserts are big and oases small. Both history and decision theory suggest that predictive validity is under-managed in drug R&D, not least because it is so hard to measure before projects succeed or fail later in the process. This article explains the influence of predictive validity on R&D productivity and discusses methods to evaluate and improve it, with the aim of supporting the application of more effective decision tools and catalysing investment in their creation.” -Jack Scanell et al.
Why is progress in biology so slow? by Sam Rodriques
“Biology is complex, and the goal of biomedical research is to cure disease. However, most people don’t take it seriously. To achieve success in curing disease like how AI has had success in other fields, three main components need to be addressed: speed, knowledge, and talent.
- Speed involves regulatory work to enable faster experiments to be done on humans.
- Knowledge involves eliminating the phenomenon where a biologist discovers something that has already been known for years. In addition, the biomedical literature needs to be more reliable.
- Talent requires creating a career path for top biology researchers so they can continue to do research instead of becoming managers. Finally, use machine learning to predict drug safety, and then work with regulators to enable drugs that are predicted with high confidence to be safe, bioavailable, and to have good PK/PD etc. to be tested directly in human patients without other preclinical trials.”
No models are perfect, but Biotech’s are terrible
The current state of drug discovery is inadequate, as current models used in the process are not effective. Finding new drugs requires sifting through an almost infinite space of possible molecules, and this process is currently slow and expensive. This can be addressed by lowering the cost of mouse studies, which can provide more complex models with higher predictive power. This approach could accelerate the development process and improve the characterization of models.
—
How to contribute?
Laura Demings Advice for how to contribute to longevity has a ton of actionable advice also applying to biotech broadly
- “Read Molecular Biology of the Cell. Follow Nature, Science and Cell (eLife, PNAS and PLOS might be better from an Open Access perspective).”
- “Understand how the industry works: Subscribe to FierceBiotech and LifeSciVC, and read Peter Kolchinsky’s writing. Read ‘The Billion Dollar Molecule’ and ‘The Antidote’ by Barry Werth, as well as ‘Genentech: The Beginnings of Biotech’ by Sally Smith Hughes.”
- “Join an iGEM team (you can find ones in your area here) and hone your experimental skills.” https://www.ldeming.com/how-to-help
- Join a community such as Nucleate to connect with other (aspiring) biotech builders
- “Join a lab: Join an aging lab or a lab that will teach you an in-demand experimental technique (for example, a top proteomics lab). Build up your understanding of the field by reading reviews and going to local conferences. BAAM is a good conference for aging biology in the Bay Area.”
- “Join a company: If you don’t know anything about biology but want to work in a scientific role, I’d recommend building a portfolio of work by doing basic analyses on public datasets. Check out 1000 Genomes, GEO, or LINCS. Look at papers that use those databases, download a small portion of the data, and start playing around with some basic models. Even if you only reproduce an already published model, the act of working with sequencing data or molecular structures is a good start.”
- Start a company
The Entrepreneur’s Guide to a Biotech Startup by Kolchinsky (must read)
How to Build a Biotech Series by Celine Halioua
- A Framework for Ideation in Early-Stage Biotech
- Some considerations when picking the type of drug
- A few thoughts on choosing which disease to develop a drug for/a>
- Venture Capital for Bio 101
- A quick guide to writing an early-stage biotech investment memo
- The Pmarca Guide to Startups
- Ycombinator startup school related to biotech
- Preclinical Drug Development
- Basics of Life Science Patents
- The Structure of (one) Early-Stage Biotech Co
- The Cost of Science
- Basics of Regulatory Affairs
- The IND - Investigative New Drug Application
- The Basics of Phase 1 Clinical Trials and Clinical Pharmacology
Preclinical Drug Development
“Preclinical development encompasses all the steps up until you first test your candidate drug in patients or healthy volunteers. The general steps are (not necessarily all sequential):
- Target identification - what are you going to drug?
- Target validation - does modulating this target have a beneficial effect in your disease of choice?
- Hit identification - The initial suite of candidate molecules to drug your target
- Lead identification - The lead molecule from the suite of hits.
- Lead optimization - The optimization of the lead into its final form to be used in humans
- Non-GLP efficacy - Studies justifying your therapeutic hypothesis
- GLP safety & tox - Safety and efficacy studies, including provisional dosing
- Investigative New Drug application - the application to the FDA to allow you to test your drug in humans. “
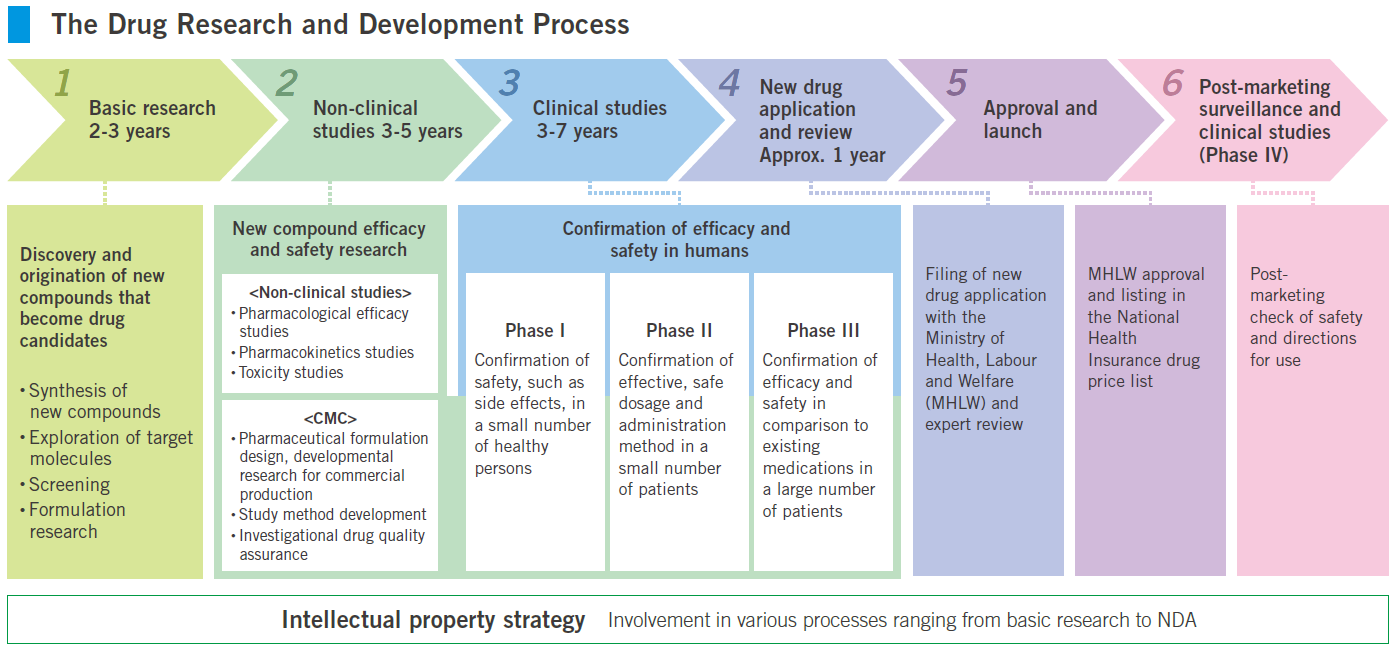
Drug research and development process

Links
- My page on AI + Life Science
- My page on longevity research
- Spinout Playbook A Guide to Spinning Out of Academia by 50y
- How to Build a Biotech by Celine Halioua
- Step-By-Step Guide to Searching for IP
- Starting a Biotech Company
- A curated, open-source set of community-built resources to help founder-led biotechs learn and thrive
- Applying tech frameworks to biotech: key differences by Celine Halioua
- AI is Industrializing Discovery by a16z
- No models are perfect, but Biotech’s are terrible